|
 |
By Adam Garrie, Contributor, The MAHA Report
On Tuesday, January 27, the HHS Office of Inspector General (OIG) issued new guidance aimed at making medically necessary prescription drugs more affordable, while still safeguarding against fraud and abuse in federal health care programs.
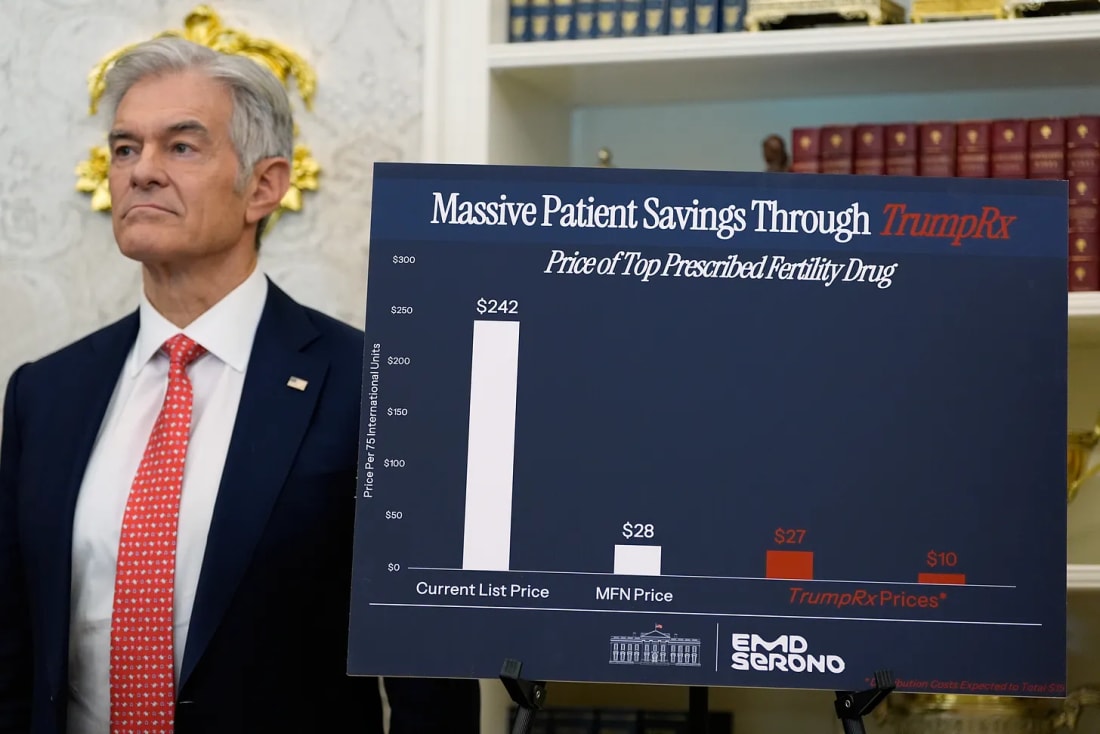
The updated guidance clears the way for pharmaceutical manufacturers to sell certain drugs directly to cash-paying customers through the forthcomingTrumpRx platform. According to HHS, direct-to-consumer (DTC) sales will help to cut costs for patients by allowing consumers to legally bypass insurers.
DTC drugs sold on TrumpRX, expected to launch later this year, will be available to all Americans, including those enrolled in Medicare and Medicaid.
HHS OIG also issued a request for information (RFI) to gather, as an HHS press release reads, “public input on creating a formal regulatory safe harbor on matters related to direct-to-consumer drug sales.”
Commenting on the new guidelines, HHS Secretary Robert F. Kennedy Jr. said, “This guidance makes clear that manufacturers can offer lower-cost drugs directly to patients without kickbacks or taxpayer billing. The Trump administration is putting patients first by increasing transparency, lowering costs, and expanding access through TrumpRx.”
Tuesday’s announcement follows months of negotiations between the White House, HHS, and major drug companies. Successful negotiations have resulted in unique most favored nations (MFN) pricing agreements through which pharmaceutical makers have agreed to, for the first time, sell their products to American consumers at the lowest prices among all developed countries.
Pfizer, AstraZeneca, EMD Serono, Eli Lilly, Novo Nordisk, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Genentech (a Roche subsidiary), Johnson & Johnson, Gilead Sciences GSK (formerly GlaxoSmithKline), Merck, Novartis, and Sanofi are among the companies that have inked MFN deals with the federal government.
“We are taking action to give patients more options while keeping strong guardrails in place,” said CMS Administrator Dr. Mehmet Oz. “When patients can access affordable medicines without hidden incentives or gamesmanship, outcomes improve and prices come down. CMS supports efforts that protect program integrity while empowering patients to make informed choices.”
Amid investigations into healthcare fraud, HHS noted that the new guidance does not change an existing federal anti-kickback statute which will remain criminally enforced.
Thank you for subscribing to The MAHA Report
You can follow us at: TheMAHA_Report on X
You can also follow us at: MAHA Action on Facebook
Make America Healthy Again™ and MAHA™ are trademarks owned by MAHA TM LLC