| From | ADEA <[email protected]> |
| Subject | ADEA Advocate - April 5, 2023 |
| Date | April 5, 2023 5:01 PM |
Links have been removed from this email. Learn more in the FAQ.
Links have been removed from this email. Learn more in the FAQ.
View this email in your browser [ [link removed] ] .
American Dental Education Association
Volume 2, No. 93, April 5, 2023
Congress Hears the Voice of Dental Education
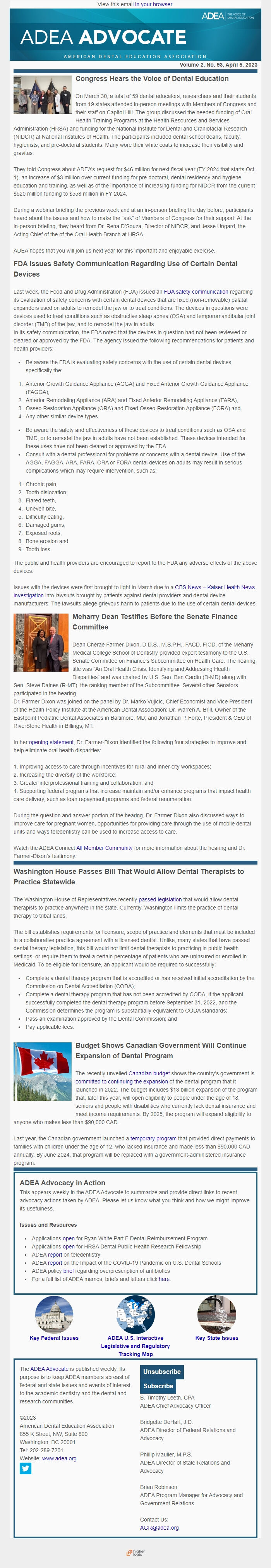
On March 30, a total of 59 dental educators, researchers and their students from 19 states attended in-person meetings with Members of Congress and their staff on Capitol Hill. The group discussed the needed funding of Oral Health Training Programs at the Health Resources and Services Administration (HRSA) and funding for the National Institute for Dental and Craniofacial Research (NIDCR) at National Institutes of Health. The participants included dental school deans, faculty, hygienists, and pre-doctoral students. Many wore their white coats to increase their visibility and gravitas.
They told Congress about ADEA’s request for $46 million for next fiscal year (FY 2024 that starts Oct. 1), an increase of $3 million over current funding for pre-doctoral, dental residency and hygiene education and training, as well as of the importance of increasing funding for NIDCR from the current $520 million funding to $558 million in FY 2024.
During a webinar briefing the previous week and at an in-person briefing the day before, participants heard about the issues and how to make the “ask” of Members of Congress for their support. At the in-person briefing, they heard from Dr. Rena D’Souza, Director of NIDCR, and Jesse Ungard, the Acting Chief of the of the Oral Health Branch at HRSA.
ADEA hopes that you will join us next year for this important and enjoyable exercise.
FDA Issues Safety Communication Regarding Use of Certain Dental Devices
Last week, the Food and Drug Administration (FDA) issued an FDA safety communication [ [link removed] ] regarding its evaluation of safety concerns with certain dental devices that are fixed (non-removable) palatal expanders used on adults to remodel the jaw or to treat conditions. The devices in questions were devices used to treat conditions such as obstructive sleep apnea (OSA) and temporomandibular joint disorder (TMD) of the jaw, and to remodel the jaw in adults.
In its safety communication, the FDA noted that the devices in question had not been reviewed or cleared or approved by the FDA. The agency issued the following recommendations for patients and health providers:
• Be aware the FDA is evaluating safety concerns with the use of certain dental devices, specifically the:
1. Anterior Growth Guidance Appliance (AGGA) and Fixed Anterior Growth Guidance Appliance (FAGGA),
2. Anterior Remodeling Appliance (ARA) and Fixed Anterior Remodeling Appliance (FARA),
3. Osseo-Restoration Appliance (ORA) and Fixed Osseo-Restoration Appliance (FORA) and
4. Any other similar device types.
• Be aware the safety and effectiveness of these devices to treat conditions such as OSA and TMD, or to remodel the jaw in adults have not been established. These devices intended for these uses have not been cleared or approved by the FDA.
• Consult with a dental professional for problems or concerns with a dental device. Use of the AGGA, FAGGA, ARA, FARA, ORA or FORA dental devices on adults may result in serious complications which may require intervention, such as:
1. Chronic pain,
2. Tooth dislocation,
3. Flared teeth,
4. Uneven bite,
5. Difficulty eating,
6. Damaged gums,
7. Exposed roots,
8. Bone erosion and
9. Tooth loss.
The public and health providers are encouraged to report to the FDA any adverse effects of the above devices.
Issues with the devices were first brought to light in March due to a CBS News – Kaiser Health News investigation [ [link removed] ] into lawsuits brought by patients against dental providers and dental device manufacturers. The lawsuits allege grievous harm to patients due to the use of certain dental devices.
Meharry Dean Testifies Before the Senate Finance Committee
Dean Cherae Farmer-Dixon, D.D.S., M.S.P.H., FACD, FICD, of the Meharry Medical College School of Dentistry provided expert testimony to the U.S. Senate Committee on Finance’s Subcommittee on Health Care. The hearing title was “An Oral Health Crisis: Identifying and Addressing Health Disparities” and was chaired by U.S. Sen. Ben Cardin (D-MD) along with Sen. Steve Daines (R-MT), the ranking member of the Subcommittee. Several other Senators participated in the hearing.
Dr. Farmer-Dixon was joined on the panel by Dr. Marko Vujicic, Chief Economist and Vice President of the Health Policy Institute at the American Dental Association; Dr. Warren A. Brill, Owner of the Eastpoint Pediatric Dental Associates in Baltimore, MD; and Jonathan P. Forte, President & CEO of RiverStone Health in Billings, MT.
In her opening statement [ [link removed] Subcommittee on Healthcare-Oral Health Crisis - Dean Farmer Dixon written testimony.pdf ] , Dr. Farmer-Dixon identified the following four strategies to improve and help eliminate oral health disparities:
1. Improving access to care through incentives for rural and inner-city workspaces;
2. Increasing the diversity of the workforce;
3. Greater interprofessional training and collaboration; and
4. Supporting federal programs that increase maintain and/or enhance programs that impact health care delivery, such as loan repayment programs and federal renumeration.
During the question and answer portion of the hearing, Dr. Farmer-Dixon also discussed ways to improve care for pregnant women, opportunities for providing care through the use of mobile dental units and ways teledentistry can be used to increase access to care.
Watch the ADEA Connect All Member Community [ [link removed] ] for more information about the hearing and Dr. Farmer-Dixon’s testimony.
Washington House Passes Bill That Would Allow Dental Therapists to Practice Statewide
The Washington House of Representatives recently passed legislation [ [link removed] ] that would allow dental therapists to practice anywhere in the state. Currently, Washington limits the practice of dental therapy to tribal lands.
The bill establishes requirements for licensure, scope of practice and elements that must be included in a collaborative practice agreement with a licensed dentist. Unlike, many states that have passed dental therapy legislation, this bill would not limit dental therapists to practicing in public health settings, or require them to treat a certain percentage of patients who are uninsured or enrolled in Medicaid. To be eligible for licensure, an applicant would be required to successfully:
• Complete a dental therapy program that is accredited or has received initial accreditation by the Commission on Dental Accreditation (CODA);
• Complete a dental therapy program that has not been accredited by CODA, if the applicant successfully completed the dental therapy program before September 31, 2022, and the Commission determines the program is substantially equivalent to CODA standards;
• Pass an examination approved by the Dental Commission; and
• Pay applicable fees.
Budget Shows Canadian Government Will Continue Expansion of Dental Program
The recently unveiled Canadian budget [ [link removed] ] shows the country’s government is committed to continuing the expansion [ [link removed] ] of the dental program that it launched in 2022. The budget includes $13 billion expansion of the program that, later this year, will open eligibility to people under the age of 18, seniors and people with disabilities who currently lack dental insurance and meet income requirements. By 2025, the program will expand eligibility to anyone who makes less than $90,000 CAD.
Last year, the Canadian government launched a temporary program [ [link removed] ] that provided direct payments to families with children under the age of 12, who lacked insurance and made less than $90,000 CAD annually. By June 2024, that program will be replaced with a government-administered insurance program.
ADEA Advocacy in Action
This appears weekly in the ADEA Advocate to summarize and provide direct links to recent advocacy actions taken by ADEA. Please let us know what you think and how we might improve its usefulness.
Issues and Resources
• Applications open [ [link removed] ] for Ryan White Part F Dental Reimbursement Program
• Applications open [ [link removed] ] for HRSA Dental Public Health Research Fellowship
• ADEA report [ [link removed] ] on teledentistry
• ADEA report [ [link removed] ] on the Impact of the COVID-19 Pandemic on U.S. Dental Schools
• ADEA policy brief [ [link removed] ] regarding overprescription of antibiotics
• For a full list of ADEA memos, briefs and letters click here [ [link removed] ] .
Key Federal Issues [ [link removed] ]
ADEA U.S. Interactive Legislative and Regulatory Tracking Map [ [link removed] ]
Key State Issues [ [link removed] ]
The ADEA Advocate [ [link removed] ] is published weekly. Its purpose is to keep ADEA members abreast of federal and state issues and events of interest to the academic dentistry and the dental and research communities.
©2023
American Dental Education Association
655 K Street, NW, Suite 800
Washington, DC 20001
Tel: 202-289-7201
Website: www.adea.org [ [link removed] ]
twitter
[[link removed]]
Unsubscribe
[link removed]
Subscribe
[link removed][0]&p_colname=p_last_nm&p_varname=p_val_arr[1]&p_colname=p_alias&p_varname=p_val_arr[2]&p_colname=p_login_id&p_varname=p_val_arr[3]&p_colname=p_passwd&p_context=NEWSLETTER&p_success_url=censsaindprofile.section_update%3Fp_profile_ty%3DINDIVIDUAL_PROFILE%26p_skip_confirm_fl%3DY%26p_section_nm%3DNewsletters%26p_format%3D110%26p_msg_txt%3D%26p_cust_id%3D%26p_referrer%3D
B. Timothy Leeth, CPA
ADEA Chief Advocacy Officer
Bridgette DeHart, J.D.
ADEA Director of Federal Relations and Advocacy
Phillip Mauller, M.P.S.
ADEA Director of State Relations and Advocacy
Brian Robinson
ADEA Program Manager for Advocacy and Government Relations
Contact Us:
[email protected] [ [link removed] ]
Powered by Higher Logic [link removed]
American Dental Education Association
Volume 2, No. 93, April 5, 2023
Congress Hears the Voice of Dental Education
On March 30, a total of 59 dental educators, researchers and their students from 19 states attended in-person meetings with Members of Congress and their staff on Capitol Hill. The group discussed the needed funding of Oral Health Training Programs at the Health Resources and Services Administration (HRSA) and funding for the National Institute for Dental and Craniofacial Research (NIDCR) at National Institutes of Health. The participants included dental school deans, faculty, hygienists, and pre-doctoral students. Many wore their white coats to increase their visibility and gravitas.
They told Congress about ADEA’s request for $46 million for next fiscal year (FY 2024 that starts Oct. 1), an increase of $3 million over current funding for pre-doctoral, dental residency and hygiene education and training, as well as of the importance of increasing funding for NIDCR from the current $520 million funding to $558 million in FY 2024.
During a webinar briefing the previous week and at an in-person briefing the day before, participants heard about the issues and how to make the “ask” of Members of Congress for their support. At the in-person briefing, they heard from Dr. Rena D’Souza, Director of NIDCR, and Jesse Ungard, the Acting Chief of the of the Oral Health Branch at HRSA.
ADEA hopes that you will join us next year for this important and enjoyable exercise.
FDA Issues Safety Communication Regarding Use of Certain Dental Devices
Last week, the Food and Drug Administration (FDA) issued an FDA safety communication [ [link removed] ] regarding its evaluation of safety concerns with certain dental devices that are fixed (non-removable) palatal expanders used on adults to remodel the jaw or to treat conditions. The devices in questions were devices used to treat conditions such as obstructive sleep apnea (OSA) and temporomandibular joint disorder (TMD) of the jaw, and to remodel the jaw in adults.
In its safety communication, the FDA noted that the devices in question had not been reviewed or cleared or approved by the FDA. The agency issued the following recommendations for patients and health providers:
• Be aware the FDA is evaluating safety concerns with the use of certain dental devices, specifically the:
1. Anterior Growth Guidance Appliance (AGGA) and Fixed Anterior Growth Guidance Appliance (FAGGA),
2. Anterior Remodeling Appliance (ARA) and Fixed Anterior Remodeling Appliance (FARA),
3. Osseo-Restoration Appliance (ORA) and Fixed Osseo-Restoration Appliance (FORA) and
4. Any other similar device types.
• Be aware the safety and effectiveness of these devices to treat conditions such as OSA and TMD, or to remodel the jaw in adults have not been established. These devices intended for these uses have not been cleared or approved by the FDA.
• Consult with a dental professional for problems or concerns with a dental device. Use of the AGGA, FAGGA, ARA, FARA, ORA or FORA dental devices on adults may result in serious complications which may require intervention, such as:
1. Chronic pain,
2. Tooth dislocation,
3. Flared teeth,
4. Uneven bite,
5. Difficulty eating,
6. Damaged gums,
7. Exposed roots,
8. Bone erosion and
9. Tooth loss.
The public and health providers are encouraged to report to the FDA any adverse effects of the above devices.
Issues with the devices were first brought to light in March due to a CBS News – Kaiser Health News investigation [ [link removed] ] into lawsuits brought by patients against dental providers and dental device manufacturers. The lawsuits allege grievous harm to patients due to the use of certain dental devices.
Meharry Dean Testifies Before the Senate Finance Committee
Dean Cherae Farmer-Dixon, D.D.S., M.S.P.H., FACD, FICD, of the Meharry Medical College School of Dentistry provided expert testimony to the U.S. Senate Committee on Finance’s Subcommittee on Health Care. The hearing title was “An Oral Health Crisis: Identifying and Addressing Health Disparities” and was chaired by U.S. Sen. Ben Cardin (D-MD) along with Sen. Steve Daines (R-MT), the ranking member of the Subcommittee. Several other Senators participated in the hearing.
Dr. Farmer-Dixon was joined on the panel by Dr. Marko Vujicic, Chief Economist and Vice President of the Health Policy Institute at the American Dental Association; Dr. Warren A. Brill, Owner of the Eastpoint Pediatric Dental Associates in Baltimore, MD; and Jonathan P. Forte, President & CEO of RiverStone Health in Billings, MT.
In her opening statement [ [link removed] Subcommittee on Healthcare-Oral Health Crisis - Dean Farmer Dixon written testimony.pdf ] , Dr. Farmer-Dixon identified the following four strategies to improve and help eliminate oral health disparities:
1. Improving access to care through incentives for rural and inner-city workspaces;
2. Increasing the diversity of the workforce;
3. Greater interprofessional training and collaboration; and
4. Supporting federal programs that increase maintain and/or enhance programs that impact health care delivery, such as loan repayment programs and federal renumeration.
During the question and answer portion of the hearing, Dr. Farmer-Dixon also discussed ways to improve care for pregnant women, opportunities for providing care through the use of mobile dental units and ways teledentistry can be used to increase access to care.
Watch the ADEA Connect All Member Community [ [link removed] ] for more information about the hearing and Dr. Farmer-Dixon’s testimony.
Washington House Passes Bill That Would Allow Dental Therapists to Practice Statewide
The Washington House of Representatives recently passed legislation [ [link removed] ] that would allow dental therapists to practice anywhere in the state. Currently, Washington limits the practice of dental therapy to tribal lands.
The bill establishes requirements for licensure, scope of practice and elements that must be included in a collaborative practice agreement with a licensed dentist. Unlike, many states that have passed dental therapy legislation, this bill would not limit dental therapists to practicing in public health settings, or require them to treat a certain percentage of patients who are uninsured or enrolled in Medicaid. To be eligible for licensure, an applicant would be required to successfully:
• Complete a dental therapy program that is accredited or has received initial accreditation by the Commission on Dental Accreditation (CODA);
• Complete a dental therapy program that has not been accredited by CODA, if the applicant successfully completed the dental therapy program before September 31, 2022, and the Commission determines the program is substantially equivalent to CODA standards;
• Pass an examination approved by the Dental Commission; and
• Pay applicable fees.
Budget Shows Canadian Government Will Continue Expansion of Dental Program
The recently unveiled Canadian budget [ [link removed] ] shows the country’s government is committed to continuing the expansion [ [link removed] ] of the dental program that it launched in 2022. The budget includes $13 billion expansion of the program that, later this year, will open eligibility to people under the age of 18, seniors and people with disabilities who currently lack dental insurance and meet income requirements. By 2025, the program will expand eligibility to anyone who makes less than $90,000 CAD.
Last year, the Canadian government launched a temporary program [ [link removed] ] that provided direct payments to families with children under the age of 12, who lacked insurance and made less than $90,000 CAD annually. By June 2024, that program will be replaced with a government-administered insurance program.
ADEA Advocacy in Action
This appears weekly in the ADEA Advocate to summarize and provide direct links to recent advocacy actions taken by ADEA. Please let us know what you think and how we might improve its usefulness.
Issues and Resources
• Applications open [ [link removed] ] for Ryan White Part F Dental Reimbursement Program
• Applications open [ [link removed] ] for HRSA Dental Public Health Research Fellowship
• ADEA report [ [link removed] ] on teledentistry
• ADEA report [ [link removed] ] on the Impact of the COVID-19 Pandemic on U.S. Dental Schools
• ADEA policy brief [ [link removed] ] regarding overprescription of antibiotics
• For a full list of ADEA memos, briefs and letters click here [ [link removed] ] .
Key Federal Issues [ [link removed] ]
ADEA U.S. Interactive Legislative and Regulatory Tracking Map [ [link removed] ]
Key State Issues [ [link removed] ]
The ADEA Advocate [ [link removed] ] is published weekly. Its purpose is to keep ADEA members abreast of federal and state issues and events of interest to the academic dentistry and the dental and research communities.
©2023
American Dental Education Association
655 K Street, NW, Suite 800
Washington, DC 20001
Tel: 202-289-7201
Website: www.adea.org [ [link removed] ]
[[link removed]]
Unsubscribe
[link removed]
Subscribe
[link removed][0]&p_colname=p_last_nm&p_varname=p_val_arr[1]&p_colname=p_alias&p_varname=p_val_arr[2]&p_colname=p_login_id&p_varname=p_val_arr[3]&p_colname=p_passwd&p_context=NEWSLETTER&p_success_url=censsaindprofile.section_update%3Fp_profile_ty%3DINDIVIDUAL_PROFILE%26p_skip_confirm_fl%3DY%26p_section_nm%3DNewsletters%26p_format%3D110%26p_msg_txt%3D%26p_cust_id%3D%26p_referrer%3D
B. Timothy Leeth, CPA
ADEA Chief Advocacy Officer
Bridgette DeHart, J.D.
ADEA Director of Federal Relations and Advocacy
Phillip Mauller, M.P.S.
ADEA Director of State Relations and Advocacy
Brian Robinson
ADEA Program Manager for Advocacy and Government Relations
Contact Us:
[email protected] [ [link removed] ]
Powered by Higher Logic [link removed]
Message Analysis
- Sender: American Dental Education Association (ADEA)
- Political Party: n/a
- Country: United States
- State/Locality: n/a
- Office: n/a
